Recently, the research team led by Professor Wang Gongke from the School of Materials Science and Engineering of our university published a research paper titled Hydrogen Bridge-Mediated Efficient Electrooxidation of 5-Hydroxymethylfurfural on Ni(OH)₂─PO₄³⁻/Ni₃(PO₄)₂ Heterojunctions in the international journal Angewandte Chemie International Edition. The first author of the paper is Associate Professor Liu Xupo, a young faculty member at the School of Materials Science and Engineering of our university. The corresponding authors are Professor Wang Gongke and Professor Wang Deli from Huazhong University of Science and Technology. Henan Normal University is the first author’s institution.
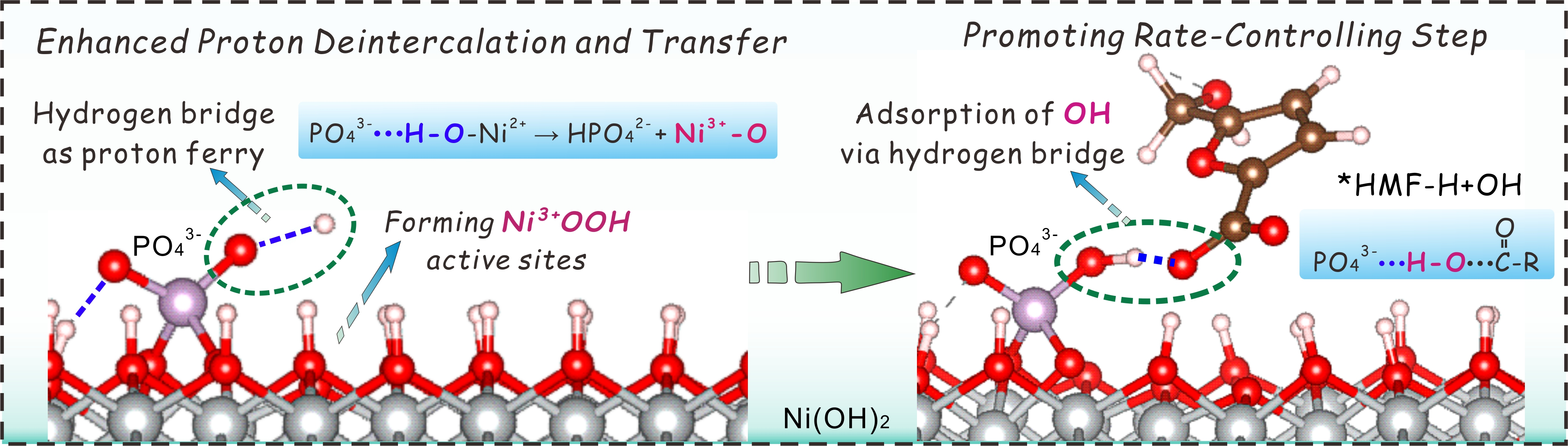
Among numerous biomass-derived molecules, 2,5-furandicarboxylic acid (FDCA) is listed as one of the top 12 most valuable biomass-derived chemicals. The electrocatalytic oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) is a green and efficient route for the production of FDCA, and accelerating proton deintercalation and transfer on thecatalyst surface is crucial for the electrooxidation of HMF to FDCA. Therefore, an in-depth investigation of the proton evolution process on the catalyst surface is of great significance for advancing research on HMF oxidation. However, most previous studies have investigated proton deintercalation and surface proton transfer separately, lacking a systematic approach that combines the two. This has, to a certain extent, impeded an in-depth understanding of the proton evolution mechanism in the HMF oxidation process. To address this challenge, the team proposed a novel hydrogen bridge-mediated mechanism for HMF electrooxidation, which utilizes phosphate groups to facilitate proton deintercalation and transfer on the catalyst surface, significantly enhancing the catalyst’s performance in HMF electrooxidation. Through a corrosion-activation strategy, they successfully synthesized a Ni(OH)₂─PO₄³⁻/Ni₃(PO₄)₂ heterojunction catalyst. The bulky PO₄³⁻ groups lead to a relatively loose and less-ordered catalyst structure, enabling the electrochemical transformation of the Ni₃(PO₄)₂ precatalyst into highly active Ni(OH)₂─PO₄³⁻ nanosheets. Density Functional Theory (DFT) calculations and pH-dependent experiments confirmed that the hydrogen bridges, induced by the PO₄³⁻ groups, promote proton deintercalation from the catalyst surface while acting as a “proton shuttle” to accelerate proton transfer efficiency. The rate-determining step of OH adsorption is achieved through a hydrogen bridge connecting the α-C atom of *HMF-H and the PO₄³⁻ group, significantly lowering the reaction energy barrier for HMF oxidation. Furthermore, the universality of the hydrogenbridge-mediated electrooxidation mechanism was verified by extending this strategy to other metal substrates such as cobalt foam, copper foam, and iron foam. This achievement marks another significant progress by our university in the catalytic conversion of biomass molecules and holds potential application prospects in the field of efficient biomass conversion.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202509274
(School of Materials Science and Engineering, Wang Liyuan, Liu Xupo)


 2025-09-04
2025-09-04



