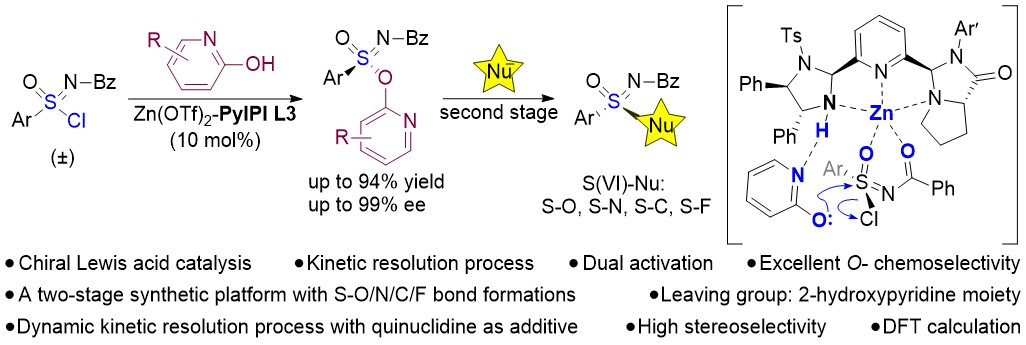
Recently, Professor Guo Haiming and Professor Xie Mingsheng from our university, in collaboration with Distinguished Researcher Tian Yin from Chengdu University of Traditional Chinese Medicine, among others, published a research paper titled Zn(II)-Catalyzed Enantioselective Nucleophilic Substitution Reactions: Access to Sulfur(VI)-Stereogenic Sulfonimidate Esters in Angewandte Chemie International Edition. Lian Saiya, a PhD student from the School of Chemistry and Chemical Engineering, is the first author of this paper. Professors Guo Haiming, Xie Mingsheng and Tian Yin are the corresponding authors, and Henan Normal University is the first corresponding affiliation.Compounds with hexavalent sulfur stereocenters are widely present in pharmaceutical molecules and biologically active molecules, and their chiral construction presents a significant challenge in the field of asymmetric catalysis. The research utilized the team's self-developed chiral tridentate pyridine N-ligand, PyIPI, to synthesize sulfonimidate esters possessing hexavalent sulfur stereocenters via Lewis acid-catalyzed asymmetric nucleophilic substitution reactions.
Experimental and theoretical computational studies revealed that the PyIPI ligand exhibits a bifunctional role: it both coordinates bidentately with the Lewis acid to activate the chlorinated sulfinimidate substrate and activates the nucleophile through hydrogen bonding. This research provides an efficient synthetic strategy for constructing hexavalent sulfur stereocenters.
This research was supported by projects from the National Natural Science Foundation of China, among others, and represents a significant advancement for our university in the field of asymmetric catalysis.
Paper Link:https://onlinelibrary.wiley.com/doi/10.1002/anie.202521905
(By Jiang Tao, Wang Manman, School of Chemistry and Chemical Engineering; Wei Ran, Office of Science and Technology)


 2025-12-18
2025-12-18



